Protein structures which have been determined using crystallography and cryoEM techniques represent only a limited range of static snapshots.
Single-molecule FRET experiments complement these techniques by revealing the full distribution of protein conformations present in solution as well as their relative abundance.
Precise FRET efficiencies obtained from these experiments can be converted to absolute distances, which allows these populations to be linked to high-resolution structures.
Distances obtained from single-molecule FRET experiments can be used to verify static structures from crystallography and cryoEM or snapshots from molecular dynamic studies (Fig 1).
smFRET experiments performed on the EI-FLEX are also not limited by protein molecular weight, solubility or crystallisability, and only require very small quantities of sample (picomolar) – which broadans the range of systems which can be analysed.
Structures provided by prediction software such as AlphaFold can now be validated with smFRET experimental data obtained at the benchtop without the need for specialist facilities.
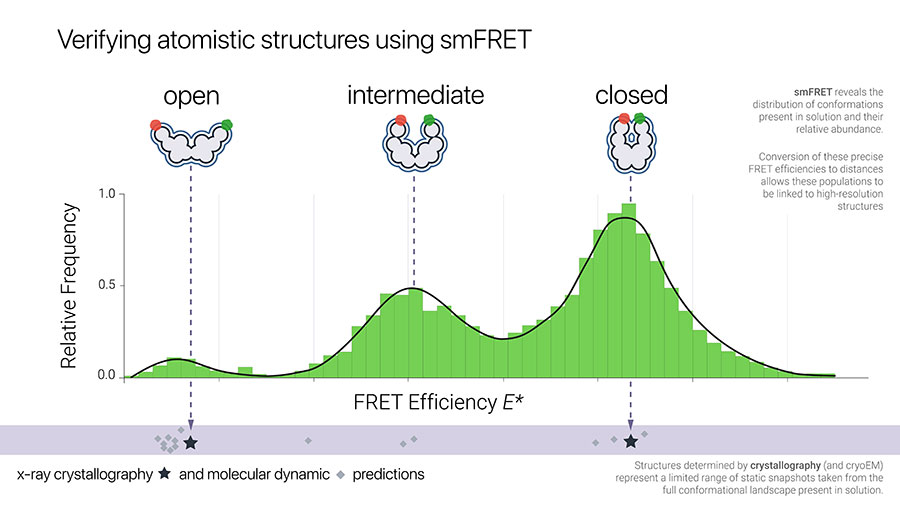 Fig 1. Correlation of smFRET effiency to absolute distance to verify structures obtained from crystallography and predictions from molecular dynamics simulations.
Fig 1. Correlation of smFRET effiency to absolute distance to verify structures obtained from crystallography and predictions from molecular dynamics simulations.
References
Craggs, T., Sustarsic, M., Plochoweitz, A., et al. Substrate conformationa; dynamics facilities structure-specific recognition of gapped DNA by DNA polymerase, Nucleic Acids Research (2019) https://doi.org/10.1093/nar/gkz797
Further reading
Craggs NAR 2019, Hugel Nat Methods 2017, Seidel Nat Methods 2012, Michaelis Nat Commun 2015